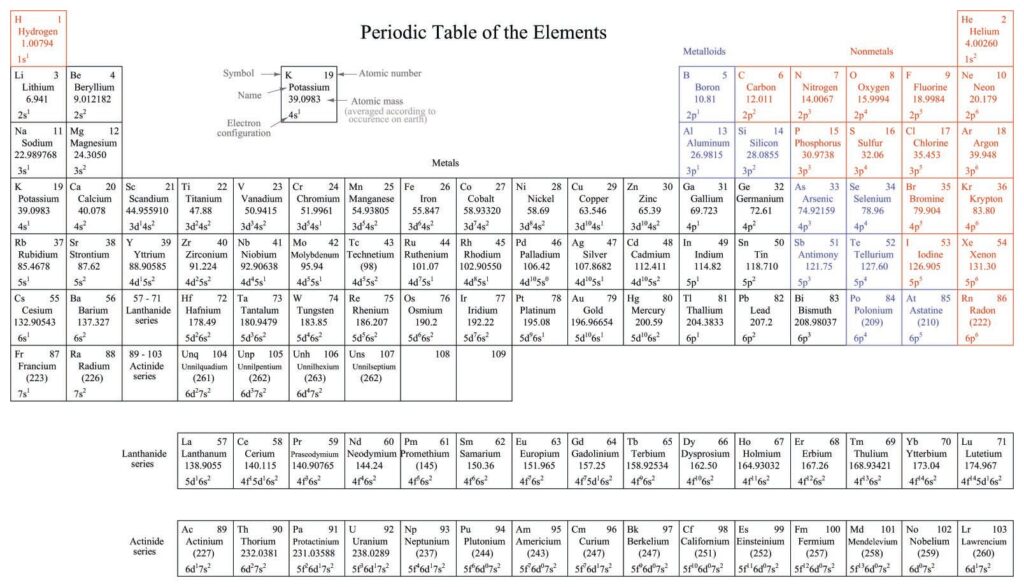
Why Are the Atomic Weights Not Whole Numbers?
In physics, one of the most fundamental questions answered is why are the atomic weights not whole numbers? The scientists have formulated and extended many theories to answer this question. In short, these theories postulate that in a closed system like our planet, the molecules, for instance, of water weigh more than the total number of the hydrogen atoms or the total number of the neutrons. The total amount of weight for each atom must be uniform across the entire volume of the atom. Thus, the atom must maintain a constant mass.
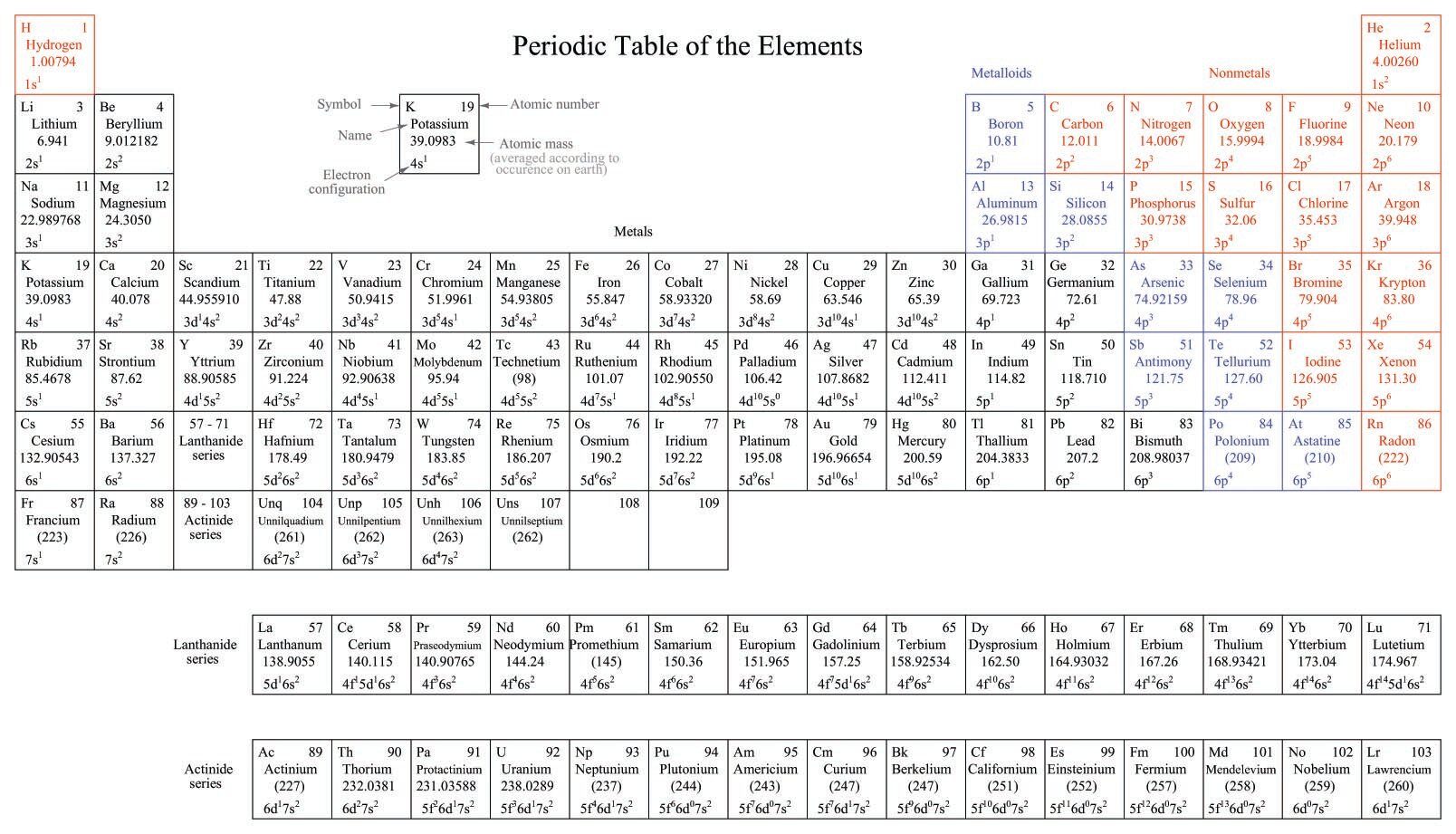
One such law/theory postulated by physicists is that the atom may lose mass because of collisions with other atoms or due to the instability of the atomic bonding. Another theory is that the bonds are self-repairing and that the losses are balanced by gains from the surrounding area. Yet another explains the unbalance through the kinetic energy of motion in the atom. A third explanation maintains that the total number of the protons is larger than the number of the neutrons. Thus, in this theory, there is an uneven balance of the number of protons that result in a smaller number of neutrons.
Most scientists believe that the observed variations in the atomic weights of elements are caused by the nuclear reaction that takes place during the formation of the elements. The rate of the reaction is dependent upon the stability of the particular element and upon the atomic number of each element. Thus, the heavier the element, the slower the reaction. The heavier the atom, the more stable it is. Thus, the atomic weights for various elements can be compared to different scales on a graph, just as they are on measuring weights.
If we look at the question from another angle, those who question the accuracy of these weights ask, “Why are the atomic weights not whole numbers?” Atoms are not made up of whole numbers, because they are not of equal amounts. In order to measure them, you would have to measure the masses of all the particles of the element. That would give you an amount that would be the mass of every atom.
There are a couple of different ways that you could do this. You could measure the number of atoms by weight, or you could measure them by the unit of measurement. If you measure by the unit of measurement, you will get a number that consists of all the unitless atoms of which the atom is a part. Each atom has a separate number that can be written down, for reference purposes, in a way that can be read off by a trained person. Thus, if you want the most accurate reading, you would use the measurement that combines both the weights and the units.
Why are the atomic weights not whole numbers? The reason is that any measurement, even a simple weight, has an underlying set of rules or measures that make it possible to calculate the value of a certain weight over a period of time. This is why almost every country in the world has a standard system of measurement, including the yardstick that is used for measuring how heavy something is, or how much something weighs. While these yardsticks vary slightly from country to country, they all use some kind of standard that is accepted throughout most parts of the world.
It is impossible, however, for any two things to be exactly the same, even for atoms. Thus, the number that you get from the graduated weighing process is actually a sum of all the smaller numbers that make up that single, complete number. The atomic weights of elements, once measured in grains of sand, were once taken as whole numbers, but the laws of physics state that anything can lose mass, but gain or lose mass in a very tiny amount, over a very short period of time. Thus, one would end up with a zero on the end of the scale, instead of a whole number.
The reason for this is that these different atomic weights essentially come from different counting procedures, and therefore, the weight readings you get are not actually one hundredths of a pound, per se, but multiplied by the amount of weight loss that occurred. So, for example, if you lost fifty pounds through your diet and exercise, you would actually weigh much less than one hundredths of a pound. Thus, you could conclude that by the logic of elementary physics, the weights on the scales we use for measuring things are not actually whole numbers, but only counting numbers, which are only one’s sum. To learn more about this topic, check out the website referenced below.